Authors
Yanlin Huang
Summary
293T and Pheonix cells grow in DMEM + 10%FBS. If you are transfecting other cells, you can use whatever medium those cells normally grow in and change to DMEM + 10%FBS on the day of the transfection. You can change back to “normal” medium 24 hours post-transfection. Calcium Phosphate transfection of M2182 in RPMI (w/o FBS) was reported to cause cells to die. All the transfection have been done in DMEM + 10% FBS, no transfection in other media was performed so far.
Materials
- Tissue culture plates with appropriate size (Fiosher).
- 293T and Pheonix cell line
- Dulbecco's Modified Eagle Medium (D-MEM) (1X), liquid (high glucose) (Invitrogen)
- Fetal bovine serum (Hyclone)
- Packaging vector PcmvR8.74
- Envelop vector PMD2VSVG
- Reporter vector DsRED, GFP or LacZ
- 2x HBS
- 2M CaCl2 (Mallinkrodt Cat # 4160)
Equipments
- Tissue Culture Incubator
Procedure
I. Day 0
- Plate 4.5x106 293T (or Pheonix for retrovirus) on 10 cm plate. Incubate overnight at 37°C. You can also scale down to 6cm or 6 well plates.
Note: alternatively, you can plate the cells the same day as the transfection. See Day 1.
II. Day 1: TRANSFECTION
Note: if you plated cells on the previous day, change medium to DMEM + 10% FBS 2 hrs prior to transfection and skip to step 4.
- Plate 293T (or Pheonix for retrovirus) at ~50% confluence on 10cm TC plates. I calculate it roughly from the original plate confluence. Ex. I will plate 2 X 10cm plates from 1 100% confluence 10cm plate. I have scaled down to 6cm and 6 well plates. Just keep final confluence of cells at roughly 50%.
- Incubate plates in 37°C incubator for ~3 to 5 hrs to allow cells to attach.
- Thaw out all reagents to room temp before proceeding with transfection.
- Once cells attached, prepare DNA mix for transfection. For each 10cm plate, add the following to 5ml polypropylene tube:
a) 10μg DNA of interested (for retrovirus, 10μg DNA)
b) 6.5μg packaging vector (packaging vector already in Pheonix, so no need to add)
c) 3.5μg envelope vector (for retrovirus, 5μg envelope vector)
d) 0.1 to 0.2μg of reporter vector (optional) – GFP, DsRED, or LacZ
e) Bring mixture up to 437.5μl with 0.1% TE in H2O
If you use different size plates, just scale down. See below table.
Note 1: You can make a master mix if you have multiple plates of the same transfection. However you need to do each transfection separately for high efficiency.
Note 2: the ratio of DNA:packaging vector: envelope vector is crucial for max viral titer. The ratio given was figured out by Weissman’s Lab. Please keep to this ratio as much as you can. - Vortex the DNA mix on highest speed setting and add 62.5μl of 2M CaCl2. While still vortexing, add 500μl 2X HBS drop wise to the DNA/CaCl2 mix (roughly 2 drops/sec using p1000 pipettor).
- Immediately add HBS/DNA solution onto cells. Do this in a gentle, drop wise manner and spread it across cells in medium.
- In a few minutes you should be able to observe, under microscope, evenly distributed small black particles on top of the cells.
- Incubate cells in 37°C incubator overnight.
III. Day 2: 24 hours post-transfection
- Change medium to 10ml fresh DMEM 10% FBS. You can generally see the efficiency of your transfection by now if you used a reporter vector. At 0.2μg ofDsRED, I can usually observe >75% transfection efficiency. If you cant see much fluorescent cells at this time, wait until 48hrs post-transfection and observe again.
Note: we have always package our viruses in 37°C incubator although it has been reported that virus is more stable if incubation is carried out at 32°C. - If you want to titer the virus, plate target cells now for infection tomorrow. I often plate 3X10 cm plates per virus for 3 different titrations.
IV. Day 3: 48 hours post-transfection
- Aliquot supernatant of transfected cells into desired volume and freeze at -80°C to kill any cells in the supernatant. Alternatively, you can filter the supethrough 0.45μm filter to remove the cells. You can also chose to centrifuge the supernatant first if there are lots of floating cells. However, you need to either freeze or filter the supe before using the virus to completely remove any chance of cell contamination.
- Store viruses at -80°C.
If you are titering or infecting target cells, follow the rest of the protocol:
Add 10μl 1000X polybrene (1000x = 5-8μg/ml) to each 10cm plate of target cells (10ml medium). Add 0.1μl, 1μl, or 10μl of virus supe to each plate of cells for titering your virus. - Incubate at 37°C overnight.
V. Day 4 : REMOVE VIRUS SUPERNATANT
- 24 hours post-infection, change to fresh medium.
- Incubate overnight at 37°C.
VI. Day 5: 24-48 HOURS POST-INFECTION
- Cells are now ready to be assay for biochemical event of interest (ex. start selection with appropriate antibiotic). The actual reverse transcription and integration take place within 24-36 hours, depending on cell growth kinetics.
- Culture cells as normal.
Recipe
- 2xHBS for calcium phosphate coprecipitation transfection:
1) Make stock sln of Na2HPO4 dibasic (5.25g in 500ml H2O)
2) Make 2xHBS:
a) 8.0g NaCl
b) 6.5g HEPES (Sigma Cat # H-7006)
c) 10ml Na2HPO4 stock solution
3) Bring volume close to 500mls. Divide into 3 batches and pH each to 6.95, 7.00, and 7.05. Test each batch using LacZ, DsRed, or GFP to see which pH gives best transfection efficiency. - 2M CaCl2:
1) Add 14.702g of CaCl2?2H2O to 50ml H2O.
2) F.W. of CaCl2·2H2O = 147.02g. -- > 2M= (2 mole/L (147.02g/mole)
3) CaCl2 is from Mallinkrodt, Cat # 4160. It is important you use their CaCl2.
References
Huang, Y. (2011). Lentivirus and Retrovirus Transfection. Bio-protocol Bio101: e38.
Stats
- Recommendations n/a n/a positive of 0 vote(s)
- Views 1536
- Comments 0
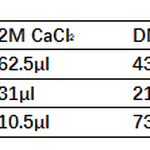 Image 1
Image 1