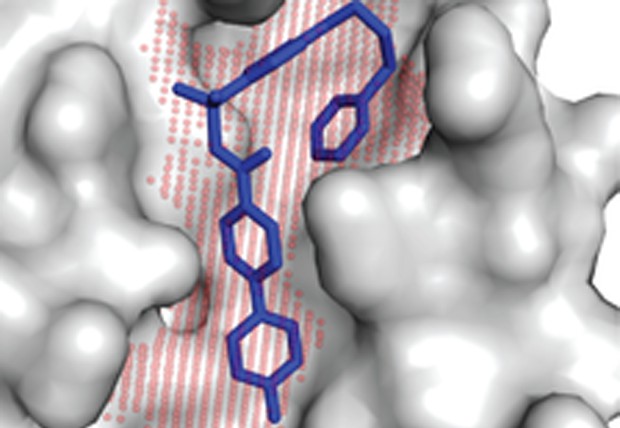
Druggable protein interaction sites are more predisposed to surface pocket formation than the rest of the protein surface, shows the study by Johnson and Karanicolas from University of Kansas.
Identifying small-molecule inhibitors of protein interactions has traditionally presented a challenge for modern screening methods, despite interest stemming from the fact that such interactions comprise the underlying mechanisms for cell proliferation, differentiation, and survival. This suggests that many protein interaction surfaces may not be intrinsically "druggable" by small molecules, and elevates in importance the few successful examples as model systems for improving our understanding of factors contributing to druggability. A new approach is now described for exploring protein fluctuations leading to surface pockets suitable for small molecule binding. The presence of such pockets is a signature of druggable protein interaction sites, suggesting that "druggability" is a property encoded on a protein surface through its propensity to form pockets. These insights may prove useful in selecting protein targets for therapeutic intervention.
Copyright: Creative Commons Attribution License.
Cover image: Modified figure from original publication.
References
Johnson DK, Karanicolas J (2013) Druggable Protein Interaction Sites Are More Predisposed to Surface Pocket Formation than the Rest of the Protein Surface. PLoS Comput Biol 9(3): e1002951. DOI: 10.1371/journal.pcbi.1002951.
Stats
- Recommendations n/a n/a positive of 0 vote(s)
- Views 591
- Comments 0