Two recent research papers suggest that an additional mechanism might control triiodothyroinine (T3) secretion in humans. Integration of in silico-analyses with SimThyr and observation of data of more than 1000 patients with and without substitution therapy with levothyroxine (L-T4) gave new hints that in humans TSH might stimulate deiodinase activity in vivo.
It is known for long time that cAMP up-regulates both type 1 and type 2 deiodinase, and it could also be shown that TSH (which uses cAMP as second messenger) is able to stimulate deiodination, but these observations resulted from animal experiments and cell culture investigations only. It was unknown if they are of any relevance in humans and on the level of the whole organism.
Combining sensitivity analysis with the open source thyroid simulation tool SimThyr and calculating total deiodinase activity (SPINA-GD) in a large cohort showed that deiodinase activity correlates with TSH concentrations, but only in patients with residual functional thyroid tissue.
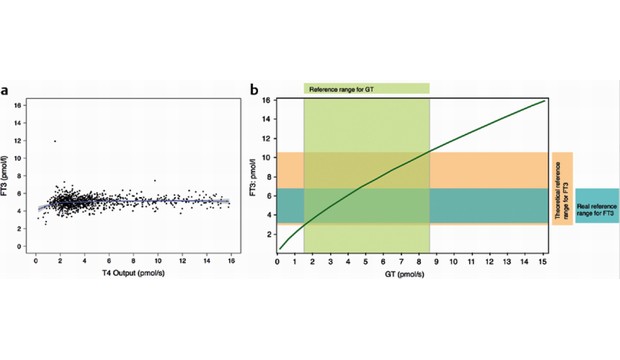
Theoretical considerations would suggest that T3 levels depend on thyroid's secretory capacity (GT) or, in substituted hypothyroidism, on daily supply with levothyroxine. Unlike computer simulations, in vivo data showed that FT3 levels remained constant over a broad range of GT or levothyroxine supply, respectively. A positive relation between L-T4 supply and FT3 levels, similar to that predicted by simulations, was seen, however, in patients with thyroid carcinoma who had received thyroidectomy and remnant ablation with high-dose radioiodine.
These results suggest the existence of a functional TSH-T3 shunt that is mainly mediated via the thyroid. This mechanism might compensate for decreasing thyroid output in beginning hypothyroidism and therefore alleviate symptoms of hypothyroidism in affected patients. Combination of computer simulations with in vivo-data helped to unveil this additional feedforward motif of thyroid homeostasis.
Cover image: Comparison of in-vivo and in-silico data (from Hoermann R, Midgley JE, Larisch R, Dietrich JW. Integration of Peripheral and Glandular Regulation of Triiodothyronine Production by Thyrotropin in Untreated and Thyroxine-Treated Subjects. Horm Metab Res. 2015 Mar 6.)
References
1: Hoermann R, Midgley JE, Giacobino A, Eckl WA, Wahl HG, Dietrich JW, Larisch R. Homeostatic equilibria between free thyroid hormones and pituitary thyrotropin are modulated by various influences including age, body mass index and treatment. Clin Endocrinol (Oxf). 2014 Dec;81(6):907-15. doi: 10.1111/cen.12527. PMID 24953754.
2: Hoermann R, Midgley JE, Larisch R, Dietrich JW. Integration of Peripheral and Glandular Regulation of Triiodothyronine Production by Thyrotropin in Untreated and Thyroxine-Treated Subjects. Horm Metab Res. 2015 Mar 6. [Epub ahead of print]. doi: 10.1055/s-0034-1398616. PMID 25750078.
Stats
- Recommendations +1 100% positive of 1 vote(s)
- Views 1107
- Comments 0
Recommended
-

Johannes W. Dietrich
Adjunct Professor | Medical Faculty, Ruhr University Bochum
Also:
- Elected member | Leibniz Society of Sciences
- Member | Center for Thyroid Medicine Ruhr University Bochum
- Head | Zentrum für Diabetes-Technologie (ZDT) Blankenstein Hospital
- Head | Zentrum für seltene endokrine Erkrankungen / Centre for Rare Endocrine Diseases Ruhr University Bochum
- Head | Diabetes Centre Bochum/Hattingen Blankenstein Hospital
- Head | Sektion Diabetologie, Endokrinologie und Stoffwechsel Ruhr University Bochum
- Collaborator | CeSER - Centrum für Seltene Erkrankungen (ZSE) der Ruhr-Universität Bochum und der Universität Witten-Herdecke Ruhr University Bochum
- Medical Advisor | Thyroid UK
- Clinical cooperation partner and statistical advisor | KreLo Medical Diagnostics
- Cofounder, Shareholder | INSTRUCT AG