Researchers at the University of Copenhagen can radically alter the properties of proteins by redesigning their chemical structure. New fundamental research based on designer proteins highlights important communication processes in the human body. In the long term, this new knowledge may lead to pharmaceuticals with fewer side effects.
Proteins play a fundamental role in almost all biological processes. They consist of chains constructed of up to 20 different amino acids, and their composition, structure and function are controlled by the genetic code. Researchers are now attempting to rewrite the core function of proteins by making alterations in their molecular backbone, for example. By combining biological and chemical methods, they are able to design semi-synthetic proteins with almost no regard to the limitations of nature:
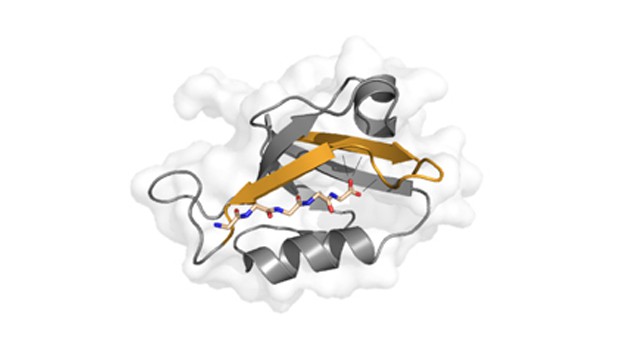
“Proteins can be regulated to perform specific biochemical tasks. We have used a technology that allows us to make changes to the molecular backbone of the protein and have thus created 22 completely new designer proteins on the basis of recognised material. Using advanced chemical-biological methods, we made the minuscule changes to the backbone of one of the most frequently occurring protein domains – a kind of fixed, independent module that features in a range of proteins. We then examined how the designer proteins bind to other proteins in the body, which allowed us to analyse the role of the specific protein domain in the body’s vital signal processes,” relates Søren W. Pedersen, postdoc.
All the cells in the body communicate via receptor proteins that are located in the cell membrane. This activates proteins inside the cell, causing specific effects which, in turn, stimulate the body to execute a variety of functions.
“Our designer proteins show us precisely how and where a bond is formed. This provides unique molecular understanding of the protein domain and a number of key protein bonds in the organism. For example the designer proteins bind to a range of receptors in the body – receptor interactions that are important targets for pharmaceuticals intended to treat stroke, pain and depression. The new findings mean that in the long term, we will be able to design pharmaceuticals that bind more strongly and more accurately to specific sites in the organism.”
From ‘on/off switch’ to ‘advanced dimmer’
Pharmaceuticals generally function by binding to a specific receptor that is involved in a given illness process – thus halting the damaging effect. Unfortunately, this strategy often generates side effects as the process simultaneously shuts down a number of beneficial functions. In recent years, interest has therefore arisen in a different strategy in which the pharmaceutical does not affect the receptor directly, but alters the interactions that the receptor has with proteins inside the cell. In other words, instead of simply switching the function of the receptor on and off, the objective is now to control parts of the receptor’s effects. It is here that designer proteins can come to play a key role:
“The capacity to manipulate proteins has led to important breakthroughs in biotechnology and biomedicine. Proteins can often target specific processes in the cells with a high degree of accuracy – and at the Center for Biopharmaceuticals we are combining chemical synthesis and biological processes to find out more about the molecular interactions that may be of significance to biological pharmaceuticals,” explains Professor Kristian Strømgaard, head of the Center for Biopharmaceuticals at the University of Copenhagen.
Content: University of Copenhagen press release (modified).
Cover image: Properties of proteins can be radically altered by redesigning their chemical structure (press release).
References
DOI: 10.1038/ncomms4215.
Stats
- Recommendations n/a n/a positive of 0 vote(s)
- Views 2616
- Comments 1
Recommended
Post a comment
Comments
-
Mike Brown Saturday, 01 February 2014 - 19:00 UTC
It amazes me how there is a massive gap between TRUE protein structure, and what scientists constantly study. Nearly 100% of membrane proteins are glycosylated, and there is hordes of papers out there that illustrate how critical protein glycosylation is for function. You can not get a proper picture of any membrane protein without considering glycosylation. Unfortnately, you can not control glycosylation because 1.) no one has figured out the "glyco code" and 2.) there is no template for control.
For example, consider ion channels, which are critical for neuron and cardiac physiology. 30% of the entire molecular weight of ion channels comes from their carbohydrate structures that are attached to them, yet if you look in the literature almost every single model or study of ion channels completely ignores ion channel glycosylation. However, if you dig deep, people have shown that ion channel glycosylation SIGNFICANTLY alters the electrical activity of an ion channel. How many peoples' models of ion channels are flat out wrong or severely lacking because they ignore glycosylation?
How about GPCRs, which comprise 50% of all targets for current medications? Almost every time people try to study or model a GPCR they completely ignore their glycosylation. End result: huge failure between models and real life.
Biologics are an emerging huge field of pharmaceutics. Sure, change protein structure, but you can still fail if you don't produce the right type of protein glycosylation. mABs can ellict severe immune response, have terrible half life, or simply won't work at all if you just change one sugar pattern on them.
There's so much we don't know and can't control that doesn't involve DNA/protein. Sure you manipulated the protein, but how about its glycosylation? If you change the amino acid sequence of a protein, you may not be able to glycosylate that protein correctly, because there are absolutely certain AA motifs where proteins are glycosylated (N-linked glycosylation). Other forms of glycosylation (O-linked, O-glcnac modfication) have no known motifs, thus you can't predict very well what would happen if you changed AA structure and if you would eliminate critical O-linked glycosylation glycoforms of your protein since there are no known motifs. The end result of a modified protein that doesn't have correct glycosylation could be one that doesn't work for all of your efforts.